The Chief Operating Officer of the NIHR Clinical Research Network (CRN) Greater Manchester has volunteered to take part in a COVID-19 booster vaccine study.
CRN Greater Manchester supports NHS, public health and social care settings to carry out research studies across Greater Manchester, East Cheshire and East Lancashire.
Sarah Fallon volunteered for the study which is trialling an Oxford/AstraZeneca variant vaccine aimed at preventing the Beta variant.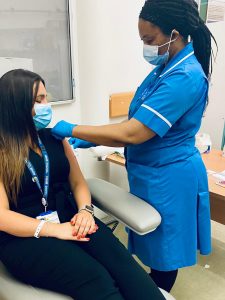
The University of Oxford, in partnership with AstraZeneca, are leading the Phase II/III study, which is supported by NIHR. The research will assess the safety and immunogenicity (the body’s ability to develop an immune response) of the new variant vaccine.
Since autumn 2020, CRN Greater Manchester has been supporting the delivery of numerous studies to identify different COVID-19 vaccines to help ensure everyone is protected against coronavirus.
Over 2,800 participants from across Greater Manchester and East Cheshire have taken part in these vaccine studies so far and Sarah wanted to do her bit as well.
Sarah took part in the study at Manchester University NHS Foundation Trust (MFT), where the study is being delivered by the Trust’s Research and Innovation Vaccine Team within the NIHR Manchester Clinical Research Facility (CRF). She had her jab at the NIHR Manchester CRF at Manchester Royal Infirmary on 9 July 2021.
Sarah, who became Chief Operating Officer at CRN Greater Manchester in 2020, was very happy to volunteer and is encouraging other people to consider getting involved in research into coronavirus.
Various studies, ranging from surveys on experiences of the pandemic to clinical trials for vaccines, are being undertaken in the region and adults can register their interest by signing up to the local NHS initiative Research for the Future.
Sarah said: “Every year we are incredibly grateful to the tens of thousands of people in Greater Manchester who show such altruism by volunteering to be part of research which helps improve care for everyone.
“So when I saw the opportunity to be part of this study, I was delighted to ‘practice what I preach’ and take part in this research which will provide vital evidence on whether booster vaccine doses, including ‘tweaks’ against new variants of COVID-19, may be needed in the future. The staff at the CRF were fantastic and I feel privileged to be involved.”
MFT is one of 14 sites across the country to be delivering the Oxford/AstraZeneca vaccine booster study. Around 1,865 adults aged 30 and above across the UK, South Africa, Brazil and Poland will be taking part.
The new variant vaccine, known as AZD2816, has been designed using the same adenoviral vector platform developed by researchers at the University of Oxford and implemented in the original Oxford/AstraZeneca COVID-19 vaccine – but with 10 minor genetic alterations to the spike protein based on the Beta variant.
The variant vaccine is being administered as a single-dose booster to study participants, including Sarah, who have previously been fully vaccinated with two doses of an approved COVID-19 vaccine. This booster dose must be at least three months after their last injection.
The study is also recruiting people who have never received a COVID-19 vaccine and these participants will receive two doses of the trial variant vaccine.
The study is recruiting participants until August, with initial data from the trial expected later this year.
Once available, data will be submitted to regulators for assessment as a next-generation booster vaccine and through an expedited regulatory pathway.
Professor Andrew Ustianowski is the Principal Investigator for the study at MFT. He is also NIHR Clinical Lead for the COVID-19 Vaccination Programme and Joint National Infection Specialty Lead.
He said: “Throughout the pandemic the UK has demonstrated its expertise in clinical vaccine research, consistently supported by the fantastic efforts of our study participants in Greater Manchester and across the country.
“The latest booster study from Oxford/AstraZeneca is just one of the latest, world-leading steps in our battle to tackle the virus and one of the variants of concern.
“We are calling on the general public once again to work alongside researchers to help recruit to this study and help gather the data we need on the new vaccine. We are extremely grateful to Sarah and all study participants.”