An NHS worker from Manchester University NHS Foundation Trust (MFT) has become the first person in the world to be consented into another leading phase 3 COVID-19 vaccine study, testing the safety and effectiveness of a new two-dose vaccine regimen developed by The Janssen Pharmaceutical Companies of Johnson & Johnson.
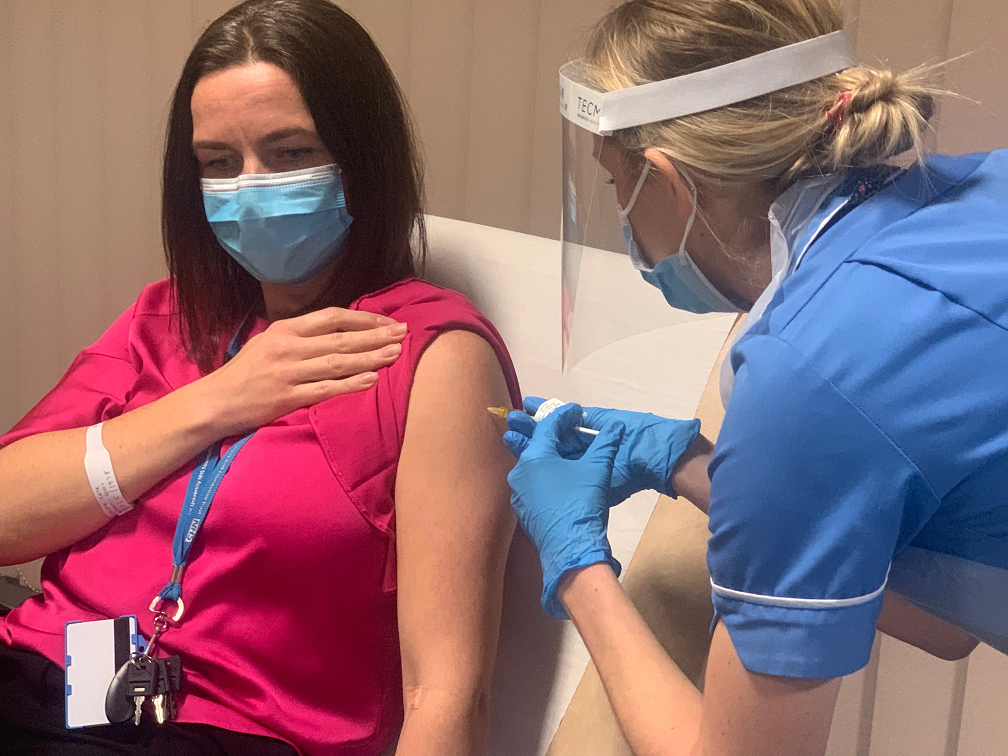 Dr Claire Cole, the Head of Research Delivery at MFT – one of the sites where the Janssen Phase 3 trial is being conducted in the UK – was consented into the trial at the site, before later also becoming one of the first in the trial to receive their first dose. Claire was the first of 6,000 volunteers in the UK, and of 30,000 worldwide, to be consented into the trial as researchers around the world continue to work to secure a range of vaccines to help tackle coronavirus.
Dr Claire Cole, the Head of Research Delivery at MFT – one of the sites where the Janssen Phase 3 trial is being conducted in the UK – was consented into the trial at the site, before later also becoming one of the first in the trial to receive their first dose. Claire was the first of 6,000 volunteers in the UK, and of 30,000 worldwide, to be consented into the trial as researchers around the world continue to work to secure a range of vaccines to help tackle coronavirus.
Claire said: “Although I have worked in health research for a number of years, I never cease to be amazed by the life-changing, and sometimes lifesaving, impact research can have. This has never been truer than during the COVID-19 pandemic, where I have seen first-hand how rapidly clinical research can be translated into treatments for our patients.
“I wholeheartedly believe in the importance of taking part in research and am honoured to be the first person in the world to be recruited to the study, and one of the first to receive the vaccine as part of this vitally important coronavirus vaccine trial.”
Volunteers from a variety of age groups and backgrounds, including some of the thousands who have registered to be contacted about vaccine studies through the NHS COVID-19 Vaccine Research Registry, have begun taking part in the latest study at 17 National Institute for Health Research (NIHR) sites across the UK, including MFT. Recruitment into the study will complete in March 2021 and the study will last for 12 months, with participants being monitored for 24 months after vaccination.
The trial, co-funded by the UK Government’s Vaccine Taskforce, is being delivered in collaboration with the NIHR Clinical Research Network Greater Manchester (CRN GM).
With several more phase 3 studies for potential vaccines expected to start over the next six months, researchers are highlighting the need for volunteers from across the UK to continue to join the fight against coronavirus by taking part in clinical studies by informed choice. In particular, they are encouraging more frontline workers, as well as volunteers from Black, Asian and ethnic minority backgrounds, to join vaccine studies.
Dr Tim Felton, Clinical Lead for all COVID-19 research at MFT and the Principal Investigator at MFT for the Janssen Phase 3 study, said:
“Throughout all the research we have undertaken into COVID-19 at MFT, finding a safe and effective vaccine has been the top priority. Delivering the first dose as part of this vaccine study is another step forward in our fight against coronavirus.
“It is critical that we explore a range of vaccination options to give us the greatest chance of protecting as many people as possible. To ensure we know that the vaccines are safe and effective we need thousands of people to sign-up to the Vaccines Registry and take part in research.”
Professor Neil Hanley, Group Director for Research and Innovation at MFT, said:
“Cutting-edge research and innovation are fundamentally about delivering when it really matters, when there are national and international emergencies and we don’t have the answers ready-made and sat waiting for us.
“MFT’s history of research expertise and excellence, coupled with our world-leading infrastructure has enabled us to be chosen as one of the UK sites to deliver this vital vaccine study that could help form part of the solution to this global health crisis.”
Sir Michael Deegan, MFT Group Chief Executive, said:
“We are immensely proud of the positive impact MFT’s research and innovation has had on our patients, both locally in Greater Manchester, and around the world – particularly in our response to COVID-19 where research at our hospitals has contributed massively to the global understanding of the coronavirus, and the care and treatments for it. We hope that the Janssen Phase 3 study running at MFT will provide the results that we are all anticipating, but while the research is taking place we must continue to follow the guidance to control the virus, protect the NHS, and save lives.”
Chair of the Government’s Vaccine Taskforce, Kate Bingham said:
“The recent news about progress on the search for a vaccine is enormously exciting for the whole world, but we must not take our focus off continuing the important research to work out which vaccines work best for different people to provide long lasting, effective protection against COVID-19.
“Many vaccines are needed both here in the UK, and globally, to ensure we can provide a safe and effective vaccine for the whole population. That’s why the launch of this trial to establish the safety, effectiveness, and very importantly the durability, of the Janssen vaccines is so significant, and I would continue to encourage people to sign up to take part in vaccine trials.
“By co-finding this study we are helping generate date for future regulatory submissions internationally as well as for the UK.”
The UK public can support the national effort to speed up vaccine research and receive more information about volunteering for clinical studies by visiting www.nhs.uk/researchcontact to join the NHS Covid-19 Vaccine Research Registry.