January 2023
Since NICE Myeloma: diagnosis and management guidelines were published and updated in October 2018, the use of serum free light chains (sFLC) in the diagnosis and monitoring of myeloma and monoclonal gammopathy of undetermined significance (MGUS) has increased exponentially. Unfortunately, sFLC tests can be difficult to interpret and often have non-specific variation from the normal reference range. The NICE guidelines recommend testing sFLC assessment in anyone with suspected myeloma and for prognostic assessment in those with myeloma and MGUS. Myeloma UK has published two very convenient guides for primary care on Myeloma and MGUS:
- Myeloma UK Myeloma and MGUS Guide for GPs: Myeloma and MGUS – A Guide for GPs
- Myeloma UK Myeloma diagnostic tool: Myeloma Diagnostic Tool: Guidance for Primary Care
Rapid assessment of patients with suspected myeloma can have a huge impact on the outcome of treatment. The Myeloma diagnostic tool provides a simple guide to when to suspect myeloma, broken down into blood test abnormalities and clinical features.
Key blood test abnormalities include:
- Raised calcium
- Renal impairment
- Anaemia
- Raised ESR
Key clinical features include:
- Bone / back pain
- Generally being unwell (fatigue / weight loss)
- Recurring infections
- Pathological fractures
- Unexplained breathlessness
Unexplained or multiple of these features raise the suspicion of myeloma and should lead to further investigations. The further investigations recommended include:
- Serum immunoglobulins and protein electrophoresis (looking for a paraprotein)
- Serum immunofixation (if a paraprotein is identified) to confirm the type of paraprotein: this test will be performed automatically by the laboratory when required
- sFLC (if not available urine Bence Jones protein)
- Full blood count
- Corrected serum calcium
- Serum creatinine
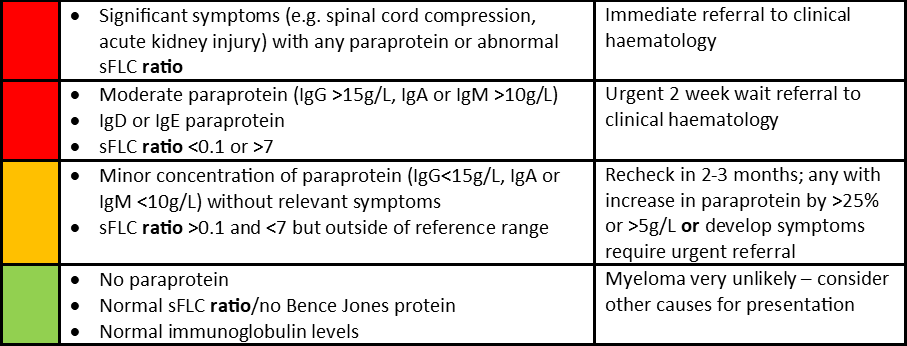
The Myeloma diagnostic tool then provides a traffic light approach to the clinical and laboratory findings as below:
Although this guide is extremely useful in assessment for myeloma there are other result patterns particularly on sFLC assessment that can appear abnormal. Each sFLC report includes a result for the level of the Kappa and Lambda light chains and for the ratio of Kappa to Lambda. The results pattern and interpretation we apply are detailed in the table below, which mirrors the recommendations from Myeloma UK:
| Result | Interpretation |
| Kappa, Lambda and ratio all within reference range | Normal/no comment provided |
| Kappa and/or Lambda outside of reference range, but ratio within range | Likely causes include inflammation or abnormal renal function |
| sFLC ratio 1.66 to 3.1
(within expected range in CKD) |
sFLC ratio falls within the reference range for CKD. In the presence of CKD no further action is required based on this result
In the absence of renal disease consider MGUS, myeloma or amyloidosis. Recommend discussion with or referral to clinical haematology if any clinical features of myeloma. In the absence of any other features of myeloma recheck with immunoglobulins and electrophoresis in 2-3 months and refer to clinical haematology if >25% change. Refer urgently if any symptoms of myeloma develop |
| sFLC ratio ≥0.1 and ≤7 but outside of reference range | Abnormality in sFLC ratio. Consider MGUS, myeloma or amyloidosis. Recommend discussion with or referral to clinical haematology if any clinical features of myeloma. In the absence of any other features of myeloma recheck with immunoglobulins and electrophoresis in 2-3 months and refer to clinical haematology if >25% change. Refer urgently if any symptoms of myeloma develop |
| sFLC ratio <0.1 or >7 | Significant abnormality in sFLC ratio. Recommend urgent 2 week wait referral to clinical haematology |
Many calls to the laboratory are related to minor abnormalities in the levels of Kappa or Lambda with a ratio within the reference range. In these cases, unless there are clear clinical features of myeloma, we would simply advise assessing the patient for inflammation/infection or renal disease. Similarly, when the ratio is mildly deranged, and in the absence of clear clinical features of myeloma, we would advise monitoring/repeat analysis after a period of 2-3 months.
References/links:
- NICE guidance: Overview | Myeloma: diagnosis and management | Guidance | NICE
- Myeloma UK Myeloma and MGUS Guide for GPs: Myeloma and MGUS – A Guide for GPs
- Myeloma UK Myeloma diagnostic tool: Myeloma Diagnostic Tool: Guidance for Primary Care