Electronic requesting must be used to request pathology investigations where it is available. Handwritten requests are more prone to sample labelling errors, transcription errors, sample collection errors and reporting errors. Do not use hand written requests unless absolutely necessary.
Handwritten request forms are accepted in Histology because these have been designed to allow annotation of anatomical diagrams.
Each request accepted by the laboratory for examination(s) shall be considered an agreement.
What to do when there is an IT failure
When there is a failure of the electronic requesting system and it is not possible to request pathology tests electronically, handwritten request forms can be used.
Critical/urgent results will be telephoned by Laboratory staff to the requesting departments. Please leave the Laboratory phone lines free for us to do this and do not contact us unnecessarily during these times.
Sample transportation
Each sample or set of samples must be placed in the plastic bag that accompanies the request form. This bag must be sealed to prevent any leakage or loss of samples in transit. Please do not use staples to seal these bags. Please ensure that the request sticker is placed on the paper form that is attached to the sample bag and NOT placed directly on to the plastic bag.
Samples must NOT be stored but should be sent to the laboratory immediately via porter or air tube or, for off-site users, by the next available transport. Users must consider the time of the next transport as delays may compromise certain results – if unsure, contact the relevant department.
Hospital requirements
Delivery in person to laboratory:
Samples must be sealed in plastic bags and must also be placed in an appropriate carrier, e.g. sturdy carry box, sealed strong bag or other approved container whilst being carried to the laboratory.
Pneumatic tube:
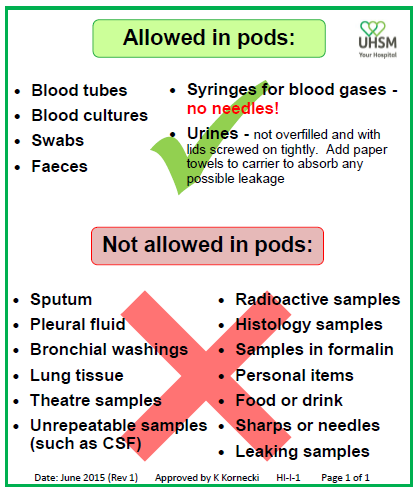
Samples in sealed bags may be placed directly into the pods and sent through the system.
GP requirements
Samples collected from GP practices are gathered into strong polythene bags which are sealed. The hospital transport drivers place these bags in the secure rigid sample transport boxes with sealable lids that they carry in their vans.
These boxes must be labelled as “Diagnostic Specimens – UN3373” and have the department and hospital name and contact telephone number.
Postal samples
Samples that are sent via the Royal Mail must be packed in special containers purchased from the Royal Mail that conform to regulation UN No 3373 – Packing instructions for Diagnostic specimens and Infectious substances (Packing instruction P650). This states that the ‘packaging must be of good quality, strong enough to withstand the shocks and loadings normally encountered during carriage’.
Sample reception
Pathology sample reception is open 24 hours a day, every day. Samples can be delivered directly to the hatch at specimen reception at the front of the Clinical Sciences Building or sent via the pneumatic tube.
Sending respiratory samples from clinical areas (including COVID swabs):
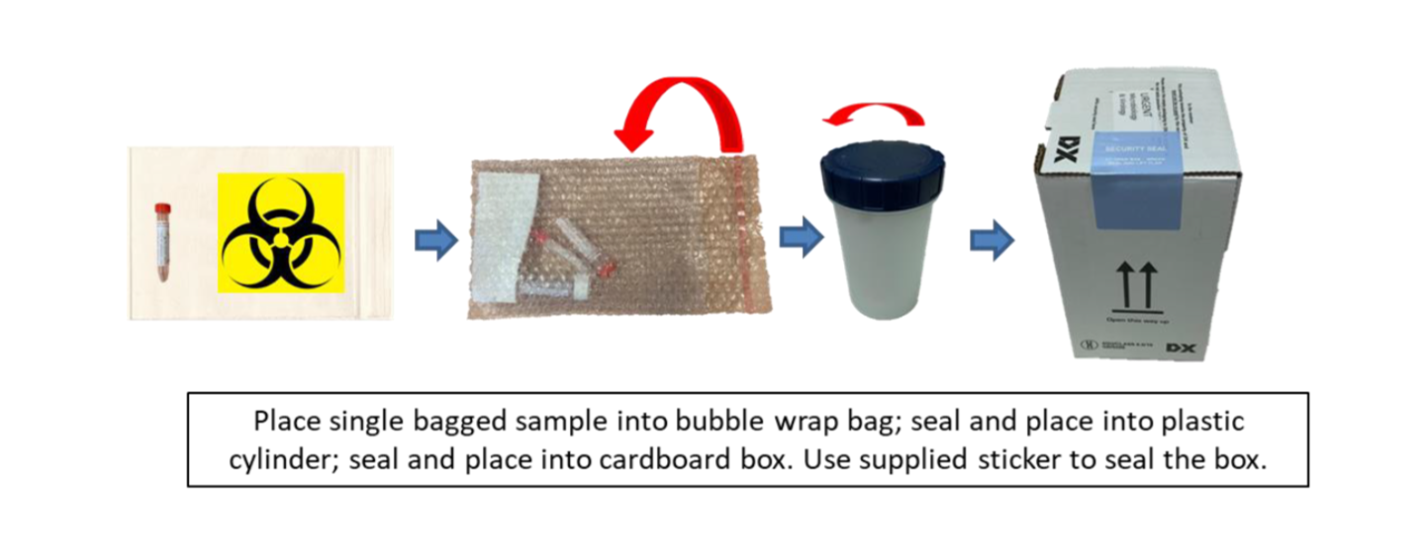
 Samples should be placed into a plastic Ziploc bag and then into another plastic Ziploc bag (preferably with a biohazard label on).
Samples should be placed into a plastic Ziploc bag and then into another plastic Ziploc bag (preferably with a biohazard label on).
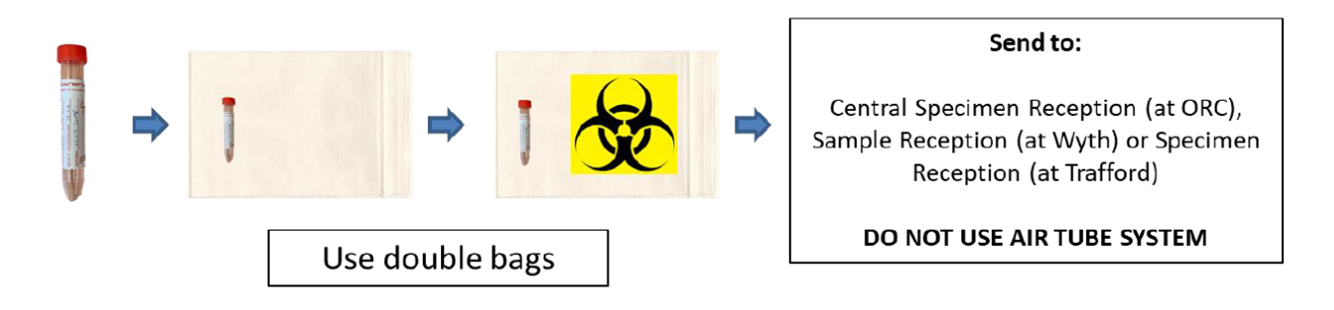
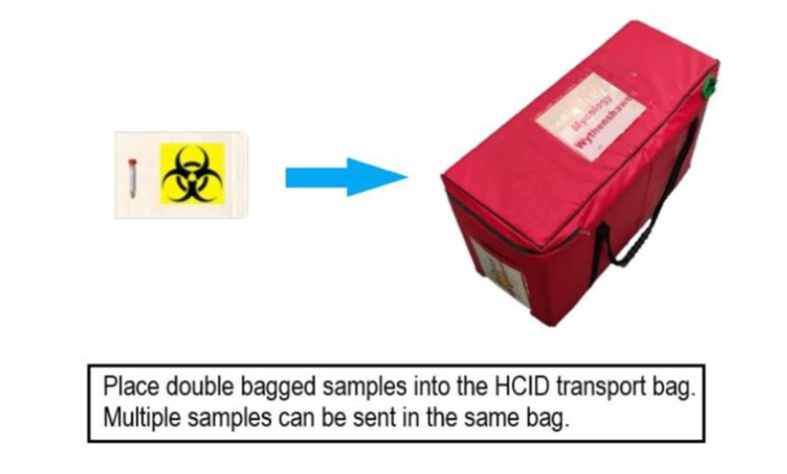
Category B transport boxes are no longer required for transport of clinical specimens from clinical areas to sample reception on the same hospital site. Samples being transported by road, between hospital sites, MUST either be placed into a Category B transport box or an appropriate transport bag (i.e. one which adheres to regulations governing the transportation of diagnostic specimens.)
Using the Cat B transport boxes:
Using the transport bags:
 Turnaround times
Turnaround times
Turnaround times that appear in these pages are from time of receipt into the laboratory information system (LIMS) to the time of reports being issued, except for histopathology turnaround times which are from the date of sample collection to date of report issue.
Where possible the time of receipt in the laboratory is recorded on the request form and corrected in the LIMS. This information is monitored monthly by each laboratory and is available on request.
(Last reviewed June 2021)
 In this section
In this section